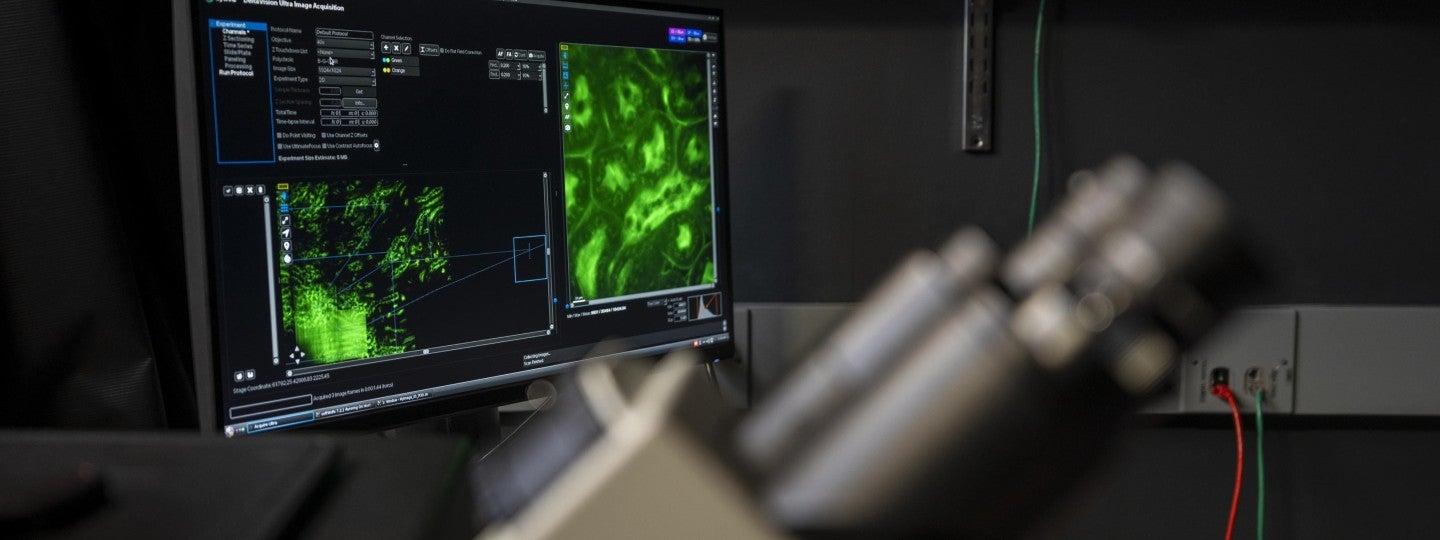
The light microscopy center within the GC3F located in 270 Klamath Hall is an interdisciplinary facility which seeks to provide centralized space, personnel, and technical tools toward advancing optical imaging in the biological sciences. The facility is designed to serve as a conduit for state-of-the-art microscopy and image analysis resources to the University of Oregon, Oregon State University, and Oregon Health & Science University communities. We offer hands-on microscopy training, experimental guidance, and image analysis training and support.
Learn about the microscopes we have available for use in the core.
Image analysis software available at our reservable workstations.
Learn how to gain training and access to the microscopy core calendar.
View recent projects completed using these shared resources.
If you have microscopy-related questions, please email Adam Fries or Doug Turnbull.
